ProQR Therapeutics Creating RNA therapies for patients in need


On a mission
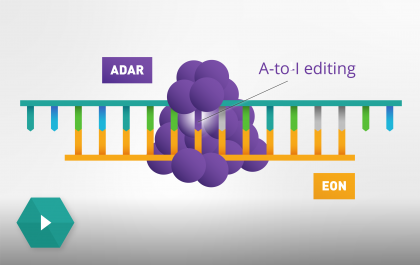
At ProQR, we are passionate about pushing the boundaries of science and technology to create innovative solutions for unmet medical needs. Our team is dedicated to conducting cutting-edge research and development in the RNA editing field to improve the lives of patients and families affected by genetic disorders.
We believe that every person deserves access to the best possible care, and we are committed to working together as a community to make that happen. Join us on our mission to make a real difference in the lives of those we serve.

Our science
Internal link Working at ProQRLatest press releases
-
Internal link ProQR Nominates Martin Maier, PhD to Board and Announces Annual Meeting of Shareholders to be Held May 22, 2024
-
Internal link ProQR Highlights Upcoming Presentations on Axiomer™ RNA Editing at ASGCT 27th Annual Meeting
-
Internal link ProQR Achieves Successful Defense of New Challenge to its Axiomer™ IP Portfolio

ProQR Community News
-
ProQR welcomes a new generation of scientists
Community news
-
Catching up with returning ProQRian René Beukema
Community news
-
Exploring why companies partner their technology
Community news
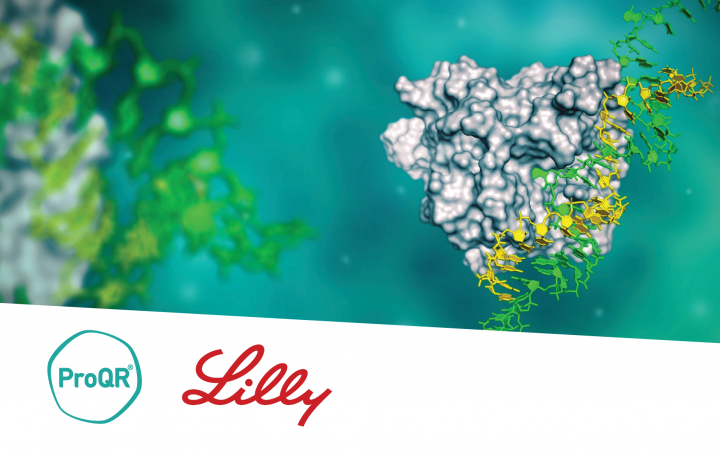
ProQR & Eli Lilly and Company partnership
Since 2021 ProQR has partnered with Eli Lilly and Company focusing on the discovery, development, and commercialization of potential new using ProQR’s proprietary Axiomer® RNA editing platform.
Internal link Read about our partnership