At ProQR, we are dedicated to improving the lives of patients and families affected by rare and common disorders using Axiomer, our innovative RNA editing platform technology.
Developing potentially life-changing medicines for patients with high unmet medical need
Cholestatic liver diseases
In cholestatic livers diseases, bile acids can’t flow properly from the liver to the gut. Excess bile acids accumulate in the liver, triggering inflammation, which can lead to scarring. The surrounding tissues, including the bile duct, can also become irritated and stop working as they should.
As the disease progresses, the liver’s ability to function deteriorates. In severe cases, this can cause irreversible damage that leads to liver failure, which requires a liver transplant. These diseases can affect individuals of all ages, yet treatment options are limited.
AX-0810 targeting NTCP
AX-0810 is an RNA editing oligonucleotide that is a potential therapy for cholestatic diseases that works by targeting cells in the liver called hepatocytes. These are the main cells that do most of the liver’s work. In these cells, AX-0810 makes a small change to the instructions (RNA) used to make a protein called NTCP. NTCP is a transport channel that lets bile acids into your liver cells.
AX-0810 helps the liver produce a slightly different version of the NTCP protein, called NTCP Q68R. This version still works well for the body, but it allows fewer bile acids into the liver. That can help reduce bile acid build-up, which could be beneficial for people with cholestatic liver diseases.
Scientists have found that some people naturally have the NTCP Q68R version from birth. These individuals report no clinical symptoms related to bile acid level concentrations.
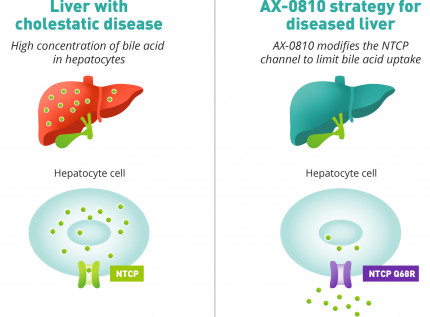
Using this approach, we aim to limit the concentration of bile acids in the liver of patients with cholestatic diseases so that the liver can recover and function properly.
Phase 1 study in healthy adult volunteers
We are planning a Phase 1 study to evaluate the safety, tolerability and biological activity of AX-0810. The study will involve healthy adult volunteers and will take place in a research center in The Netherlands once it has been approved by the health authorities. Initial data from the first-in-human study is expected in late 2025.