Science RNA technology to benefit patients


At ProQR science is literally at the heart of what we do. The offices at our headquarters in Leiden are centered around the laboratories where our scientists discover and test our novel RNA therapies. Our passion lies in discovering the latest RNA technologies and translating them to life-changing treatments for people with rare genetic diseases.
Axiomer RNA base editing
At ProQR we invented the Axiomer™ technology that enables single base editing to target currently untreatable diseases. We have partnered with Eli Lilly on this platform technology.
Internal link View this technology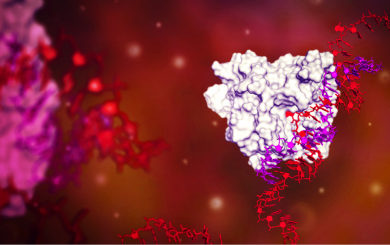
Scientific presentations and publications
Our clinical trial results and the groundbreaking science underlying our pipeline programs were published by world-class key opinion leaders as well as our scientists.
Internal link See the science
Partnerships
We are proud partners of Eli Lilly and Company who in-licensed our RNA technologies to develop new medicines to potentially address important unmet medical needs.
Internal link Learn more
Meet Gerard Platenburg, co-founder and Chief Scientific Officer at ProQR
Always excited about RNA - “I really enjoy turning new scientific ideas into a clinical reality.”
Internal link Read the interview